Ultra-pure Alginate for Medical Use
(Low Endotoxin/ Injectable-Grade Alginate)
Print
World’s First Technology
KIMICA was the first in the world to establish an alginate manufacturing facility for an injectable-grade API, successfully commercializing ultra-pure medical-grade alginate (low endotoxin / injectable-grade).
 This product is an alginate with endotoxin (pyrogen) levels sufficiently reduced to allow direct administration into the body via injection.
This product is an alginate with endotoxin (pyrogen) levels sufficiently reduced to allow direct administration into the body via injection.
The commercialization of this product has significantly expanded the application of alginate in the medical field.
The product is already used as a raw material for medical devices, and clinical trials in regenerative medicine are being actively conducted. It is a highly regarded biomaterial in advanced medical fields such as tissue engineering and regenerative therapies, helping to save lives, protecting health, and alleviating pain.
Furthermore, as this product is manufactured in compliance with Good Manufacturing Practice (GMP) for active pharmaceutical ingredients, its efficacy can be confirmed with high reproducibility from the research stage through to full-scale commercial production.
Characteristic
GMP
KIMICA holds a pharmaceutical manufacturing license at its Chiba Plant to supply alginate products API (Active Pharmaceutical Ingredients). At the Chiba Plant, alginate products are produced under GMP standards for API. KIMICA is able to supply high-quality alginates as both active pharmaceutical ingredients and medical-grade raw materials.
Low Endotoxin
As a pioneer in alginate, KIMICA has a long history and has established a technology to remove endotoxins through years of research. As a result, KIMICA offers alginate products with sufficiently reduced endotoxin levels to allow direct administration into the body via injection.
Ultra-Highly Purified
KIMICA supplies highly purified alginate products, with impurities thoroughly removed. It is also possible to perform pre-filtration through a 0.2 μm filter.

Specialized Equipment
Ultra-high purity grade sodium alginate is commercially manufactured using specialized equipment at our Chiba Plant.

Low Endotoxin
Traditionally, alginate used for medical applications has been intended for oral administration or external use on the body.
In the future, more advanced medical uses of alginate may involve direct administration into the body via injection.
In such cases, it is essential to remove impurities such as pyrogens (fever-inducing substances).
KIMICA offers low-endotoxin alginate products in which pyrogens (endotoxins) have been sufficiently reduced, making them suitable for direct administration into the body.
- What is Endotoxin?
- Endotoxins are lipopolysaccharides found in the cell walls of gram-negative bacteria. They are released when these microorganisms die and their cells break down.
When endotoxins enter the body through the bloodstream, even in very small amounts, they can cause fever, septic shock, and potentially life-threatening conditions.
Pharmaceuticals and medical devices, especially injectable products that are directly introduced into the body, are required to be endotoxin-free.
- Endotoxins in Alginate
- Alginate extracted from natural seaweed typically contains tens of thousands to hundreds of thousands of endotoxin units (EU) per gram.
While this amount poses no problem for foods or oral medications, endotoxins must be removed to very low levels from alginate used in injectable products.
Application
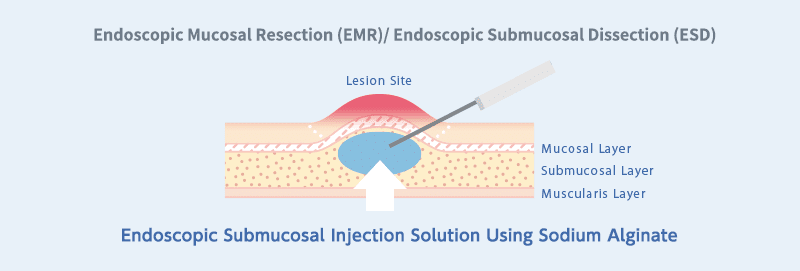
Endoscopic Submucosal Injection Solution
Sodium Alginate is used as a raw material for endoscopic submucosal injection solutions employed to lift lesions during procedures such as Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD).
Traditionally, saline solutions have been commonly used to create mucosal elevation, but the use of sodium alginate is projected to produce higher and longer-lasting elevations.
[References] Kusano T, Etoh T, Akagi T, Ueda Y, Shiroshita H, Yasuda K, Satoh M, Inomata M, Shiraishi N, Kitano S. Evaluation of 0.6% sodium alginate as a submucosal injection material in endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014 Sep;26(5):638-45. doi: 10.1111/den.12268. Epub 2014 Mar 24. PMID: 24655031.
Cartilage Repair
Studies have evaluated ultra-pure alginate as a medical device for the repair of damaged articular cartilage in joints such as the knee and elbow.
The transplanted alginate hydrogel is believed to serve as a scaffold, promoting the repair of cartilage tissue.
[References] Onodera, T., Momma, D., Matsuoka, M., Kondo, E., Suzuki, K., Inoue, M., Higano, M., & Iwasaki, N. (2023). Single-step ultra-purified alginate gel implantation in patients with knee chondral defects. The Bone & Joint Journal, 105-B (8), 880-887. doi: 10.1302/0301-620X.105B8.BJJ-2022-1071.R2
Tissue Repair of Intervertebral Discs
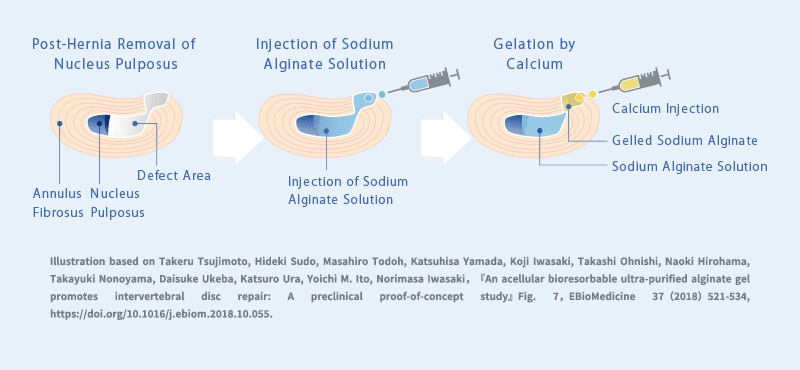
Research is underway on the use of sodium alginate for the treatment of herniated intervertebral discs.
In surgical procedures for herniated discs, the removal of herniated tissue often leaves a cavity inside the disc, which can hinder tissue repair. Postoperative pain may persist, and in some cases, disc degeneration progresses, leading to recurrence of herniation or the development of conditions such as spinal canal stenosis or degenerative spondylolisthesis, which may require additional surgery. As a result, new treatment strategies are being explored.
One such approach involves filling the cavity inside the disc with sodium alginate and sealing the injection site through gelation to prevent leakage. Studies have shown that sodium alginate can serve as a scaffold for tissue repair within the treated intervertebral disc.
[References] Katsuhisa Yamada, Takahiko Hyakumachi, Terufumi Kokabu, Kenichiro Maeda, Toshiyuki Isoe, Khin Khin Tha, Yoichi M. Ito, Takashi Ohnishi, Tsutomu Endo, Daisuke Ukeba, Hiroyuki Tachi, Yuichiro Abe, Yoko Ishikawa, Nozomi Yokota, Takashi Miyakoshi, Osamu Sugita, Norihiro Sato, Norimasa Iwasaki & Hideki Sudo, Acellular, bioresorbable, ultra-purified alginate gel implantation for intervertebral disc herniation: Phase 1/2, open-label, non-randomized clinical trials. Nat. Commun. 16, 4285 (2025). doi: 10.1038/s41467-025-59715-0
Other Applications:
Biotechnology and Medical Fields
Sheet and 3D cell culture, Cell and Tissue Preservation, Scaffolds for Tissue Regeneration, Encapsulation of Secretory Cells, Sustained Drug Release
Selection Guide (Typical Products)
Low Endotoxin Sodium Alginate
| Type | Grade | Anhydrous 1% viscosity mPa・s | Approximate Molecular weight(weight average molecular weight) | M/G | Endotoxin EU/g | TVC* cfu/g |
|---|---|---|---|---|---|---|
| Standard | AL500 | 450 - 600 | 2.15 - 2.45million | 0.8 - 1.6 | ≦50 | ≦100 |
| AL100 | 50 - 200 | 0.80 - 1.5million | 0.8 - 1.6 | ≦50 | ≦100 | |
| AL20 | 20 - 50 | 0.55 - 0.8million | 0.8 - 1.6 | ≦50 | ≦100 | |
| AL10 | 5 - 20 | 0.3 - 0.55million | 0.8 - 1.6 | ≦50 | ≦100 | |
| High-G | ALG300 | 250 - 400 | 1.65 - 2.05million | <0.8 | ≦50 | ≦100 |
| ALG100 | 50 - 200 | 0.8 - 1.5million | <0.8 | ≦50 | ≦100 | |
| ALG20 | 20 - 50 | 0.55 - 0.8million | <0.8 | ≦50 | ≦100 | |
| ALG10 | 5 - 20 | 0.3 - 0.55million | <0.8 | ≦50 | ≦100 | |
| High-M | ALM100 | 50 - 200 | 0.8 - 1.5million | >1.6 | ≦50 | ≦100 |
| ALM20 | 20 - 50 | 0.55 - 0.8million | >1.6 | ≦50 | ≦100 |
*TVC - Total viable bacterial count
Reference Viscosity
・Orange Juice 5 ~15 mPa・s
・Olive Oil ~90 mPa・s
・Sweetened Condensed Milk ~200 mPa・s
・Egg Yolk ~800 mPa・s
・Glycerin ~1,100 mPa・s
*Measured with a rotational viscometer at 20℃, 30 rpm (appropriate rotor used).
1 mPa・s = 1 cP
Inquiries about the products